PRODUCT IDENTIFICATION
H.S. CODE
Oral rat LD50: 260 mg/kg
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
Vanadium a soft, ductile, silver-grey metallic transition element in the
member of group Vb of the periodic table; symbol V; Atomic number 23; atomic
mass; 50.9415; melting point ca 1,890 C; boiling point ca 3,380 C; specific
gravity about 6 at 20 C; valence +2, +3, +4, and maximum +5; electronic config
[Ar]3d34s2; resembles chromium in properties; found in several minerals such as
carnotite, patronite, roscoelite, and vanadinite with good structural strength.
Vanadium has two natural isotopes, 50v and 51v, and several radioactive
isotopes (46-49V, 52-54V) have been obtained artificially. It dissolves in
water to form acidic solutions and dissolves in acids. It reacts with bases to
form vanadates. Vanadium trioxide (V2O3) is basic in solution and dissolves in
acids to give the green hexa-aquo ion (V(H2O)6)3+. In solution, V3+ is a strong
reducing agent and slowly attacks water with the production of hydrogen.
Vanadium is usually found bound to oxygen as a negatively charged polymeric
oxyanion that tends to complex to polarizable ligands, such as phosphorus and
sulfur. Vanadium can be obtained by the extraction from ores, extraction from
fossil fuels and extraction from slag . It has good resistance to corrosion by
hydrochloric and sulfuric acids, saltwater, or alkalies at room temperature but
oxidizes readily above 660 C. Vanadium has good structural strength and a low
fission neutron cross section, making it useful in nuclear applications. It is
intermediate between the metals and the non-metals, having either an
electronegative or an electropositive metal results in numerous chemical
compounds including vanadates and complex organic compounds. The principal use of
vanadium is in alloys, where almost three quarters of all vanadium produced is
used as additive in making special steels. It is a powerful alloying agent; a
small amount adds strength, toughness and heat resistance. It is added to steel
in the form of either ferrovanadium (vanadium-iron alloy) or vanadium carbide.
Vanadium is also a major alloying element in titanium alloys for high-strength.
Non-ferrous metals alloys are used in the atomic energy industry, aircraft and
space technology due to its low fission neutron cross section. Vanadium
disilicide is used in the production of high-temperature refractory products.
Vanadium-aluminum-titanium alloys are used in high-speed airframes and jet
engines. Vanadium chemical compounds such as vanadium oxides and various
vanadates are used as catalysts for the synthesis of sulfuric acid (the oxidation of SO2 to SO3) and maleic
and phthalic anhydride, the oxidation reaction of organic compounds
including ethanol to acetaldehyde
and
petroleum cracking. They are used in the manufacture of
polyamides (nylon), sugar to oxalic acid and anthracene to
anthraquinone. They are used as catalytic converters for the exhaust gases
of internal combustion engines. They also have applications in producing glass
and enamels, organic ion exchangers, luminescent compounds, synthetic rubbers, thermistors and switching elements as well as in producing
paints, as developers, sdepolarizers, and colouring agents in photography and
cinematography. They are used in inhibiting UV light
transmission in glass. Vanadium compounds are used as mordants in the dyeing and
printing of fabrics, particularly for fixing aniline black on silk (in form of ammonium vanadate)
and
cathode-ray tubes. Europium-activated yttrium vanadate is used in colour
television tubes. Vanadium hydride can be used as a neutron moderator in atomic
reactors. Soluble salts of arsenous-vanadous acid have been used as
fungicides and insecticides. Vanadium slags are used in casting shops as a
moulding material to improve the quality of the casting surface and to
facilitate cleaning.
- Ammonium Metavanadate [H4NO3V, CAS RN: 7803-55-6] white or slightly yellow crystals; hygroscopic.
- Ammonium Sodium Vanadium Oxide [CAS RN: 39455-80-6]
- Ammonium Vanadate [(NH4)3VO4, CAS RN: 11115-67-6]
- Ammonium Vanadium Oxide
- Bis(benzene)vanadium [C12H12V, CAS RN: 12129-72-5]
- Bis(cyclopentadienyl)vanadium dichloride [VC10H10Cl2, CAS RN: 12083-48-6] Pale green crystals
- Bismuth vanadium oxide [CAS RN: 53801-77-7]
- Ferrovanadium [CAS RN: 12604-58-9]
- Manganese Vanadium Oxide [CAS RN: 56729-39-6]
- Potassium Metavanadate [KO3V, CAS RN: 13769-43-2]
- Sodium Ammonium Vanadate [CAS RN: 39455-80-6]
- Thulium Vanadium Oxide [CAS RN: 93585-68-3]
- Sodium Metavanadate [NaVO3, CAS RN: 13718-26-8]
- Tetracarbonylcyclopentadienylvanadium [C9H5O4V, CAS RN: 12108-04-2]
- Tetravanadium Decaoxide [O10V4, CAS RN: 12503-98-9]
- Tetravanadium Octaoxide [O8V4, CAS RN: 12503-87-6]
- Vanadium Carbide [VC, CAS RN: 12070-10-9] Hard, black crystals, melting at 2800 C, boiling at 3900 C; insoluble in acids, except nitric acid; used in cutting-tool alloys and as a steel alloying agent.
- Vanadium Diboride [VB2, CAS RN: 12007-37-3]
- Vanadium Dicarbide [VC2, CAS RN: 12542-39-1]
- Vanadyl Dichloride [VOCl2, CAS RN: 10213-09-9] Toxic, green crystals, soluble in alcohol and ether; decomposes in hot water; used as a reducing agent. Also known as vanadous chloride.
- Vanadium Dimer [V2, CAS RN: 12597-60-3]
- Vanadium Dioxide [VO2, CAS RN: 12036-21-4]
- Vanadium Disilicide [VSi2, CAS RN: 12039-87-1]
- Vanadium Fluoride [VF4, CAS RN: 10049-16-8]
- Vanadium Hexacarbonyl [V(CO)6, CAS RN: 14024-00-1]
- Vanadium Isooctanoate
- Vanadium Monosulfide [VS, CAS RN: 12166-27-7]
- Vanadium Monoxide [VO, CAS RN: 12035-98-2]
- Vanadium Nitride [VN, CAS RN: 24646-85-3]
- Vanadium Octanoate [CAS RN: 74630-99-2]
- Vanadium Oxalate [CAS RN: 14974-48-2]
- Vanadium Oxide: A compound of vanadium with oxygen, for example, vanadium tetroxide (V2O4), vanadium trioxide or sesquioxide (V2O3), vanadium oxide (VO), and vanadium pentoxide (V2O5).
- Vanadium Sulfide [V2S5] A toxic, black-green powder; insoluble in water, soluble in alkalies and acids; decomposes when heated; used to make vanadium compounds. Also known as vanadic sulfide; vanadium pentasulfide.
- Vanadium Oxide Tributoxide
- Vanadium Oxychloride [VCl3O, CAS RN: 7727-18-6]
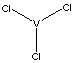
- Vanadium Oxytrichloride [CAS RN: VOCl3] A toxic, yellow liquid that dissolves or reacts with many organic substances; hydrolyzes in moisture; boils at 126 C; used as an olefin-polymerization catalyst and in organovanadium synthesis.
- Vanadium Pentoxide [CAS RN: 1314-62-1]
- Vanadium Pentoxide [V2O5, CAS RN: 1314-62-1] A toxic, yellow to red powder, soluble in alkalies and acids, slightly soluble in water; melts at 690°C; used in medicine, as a catalyst, as a ceramics coloring, for ultraviolet-resistant glass, photographic developers, textiles dyeing, and nuclear reactors. Also known as vanadic acid anhydride.
- Vanadium Silicate [CAS RN: 12653-89-3]
- Vanadium Silicide [SiV3, CAS RN: 12039-76-8]
- Vanadium Sulfide; [V2S5] Toxic, black-green powder; insoluble in water, soluble in alkalies and acids; used to make vanadium compounds.
- Vanadium Tetrabutoxide [CAS RN: 6167-45-9]
- Vanadium Tetrachloride [VCl4, CAS RN: 7632-51-1] A toxic, red liquid; soluble in ether and absolute alcohol; boils at 154°C; used in medicine and to manufacture other vanadium comounds; used as a catalyst to produce rubbers and polyolefins and as a fixative in dying.
- Vanadium Tetrafluoride [VF4, CAS RN: 10049-16-8]
- Vanadium Tetraisopropoxide
- Vanadium Tetraoxide [V2O4, CAS RN: 12036-73-6] A toxic blue-black powder; insoluble in water, soluble in alkalies and acids; melts at 1967°C; used as a catalyst.
- Vanadium Trichloride [VCl3, CAS RN: 7718-98-1] Toxic pink crystals; soluble in ether and absolute alcohol; decomposes in water and when heated; used to prepare vanadium and organovanadium compounds (esterfication and transesterfication catalyst); used as a catalyst in the polymerization of olefins, epoxy, phenolic and silicone resins.
- Vanadium Trichlorooxo
- Vanadium Trifluoride [CAS RN: 10049-12-4]
- Vanadium Trioxide [V2O3, CAS RN: 1314-36-7] Toxic, black crystals; soluble in alkalies and hydrofluoric acid, slightly soluble in water; melts at 1970 C; used as a catalyst.
- Vanadium Trichloride [VCl3] Toxic, deliquescent, pink crystals; soluble in ether and absolute alcohol; decomposes in water and when heated; used to prepare vanadium and organovanadium compounds.
- Vanadocene [VC10H10, CAS RN: 1277-47-0]
- Vanadyl Acetylacetonate [VC10H14O5, CAS RN: 3153-26-2]
- Vanadyl Chloride [V2O2Cl4] Toxic, deliquescent, water- and alcohol-soluble green crystals; used to mordant textiles.
- Vanadyl Dichloride [VOCl2, CAS RN: 10213-09-9]
- Vanadyl Naphthenate [CAS RN: 68553-60-6]
- Vanadyl Octaethylporphine [VC36H44N4, CAS RN: 27860-55-5]
- Vanadyl Oxalate [CAS RN: 15500-04-6] Used as a catalyst to remove sulfur and nitrogen in oil refinery and other chemical production processes.
- Vanadyl Etioporphyrin III [VC32H36N4O, CAS RN: 25878-85-7]
- Vanadyl Mesotetraphenylporphine [VC44H28N4, CAS RN: 14705-63-6]
- Vanadium Oxytrichloride [VOCl3 , CAS RN: 7727-18-6] Toxic, corrosive yellow liquid; emits red fumes on exposure to air; used as a catalyst to produce rubbers and polyolefins and as a mordant in dying.
- Vanadyl Phthalocyanine [VC32H16N8O, CAS RN: 13930-88-6]
- Vanadyl Sulfate [ VOSO4, CAS RN: 27774-13-6, 123334-20-3] Blue crystals, toxic, very soluble in water; used as a reducing agent, catalyst, glass and ceramics colorant, mordant and pharmaceuticals.
APPEARANCE
V CONTENT
30.8% min
CHLORIDE
64.2% min
Fe
0.1% max